





















4 – 5 November, Dubai, UAE
with attendance of Dr yaser davoudian
The vibrant city of Dubai hosted the highly anticipated Asia-Pacific Regional Meeting of the World Patient Alliance (WPA) on November 4-5, 2023. The gathering brought together global leaders from patient organizations, passionate patient advocates, and dedicated healthcare stakeholders.
A dynamic combination of face-to-face and virtual interaction: One of the outstanding features of the Asia-Pacific Regional Meeting was its hybrid format. This forward-thinking approach allowed participants to connect both in person and virtually, ensuring a diverse and inclusive experience that transcended geographic boundaries. This reflects WPA’s commitment to creating meaningful engagement for a global audience.
Insights from keynote speakers: The event featured a mix of keynote speakers from national and international health organizations, each bringing their unique perspectives to the fore. Insights shared by Mondher Letaief, Shin Ushiro, Lamya Alhazani, Candace Henley, Christiane El Ferzli, Nicole Sheahan, Kimberly Galia, and in-house speakers Andrew Spiegel Esq., Penney Cowan, and Hussain Jafri added depth to the discussions. Overview of key topics: The diverse range of topics covered at the Asia Pacific Regional Meeting provides a comprehensive look at the challenges and opportunities in patient advocacy. From “The Art of Patient Advocacy” to discussions on “Universal Health Coverage” and surveying “Health Emergencies in the Region”, each session contributed to a comprehensive understanding of the current healthcare landscape.
Panel Discussions: Expert Conversation: The event agenda was enriched with panel discussions featuring healthcare experts and patient safety champions. Anas Nofil, Ken Tanda, Edmond Love, Arij Saqer, JS Arora, Mekia Majrashi, Inge Damanti and Regina Kamuga shared their experiences and perspectives, fueling a rich conversation about the future of patient safety and healthcare. Leadership Insights: WPA leadership, including Andrew Spiegel, Penny Cowan, and Hussain Jafari, shared their insights on the success of the event. Their insights highlighted the growing role of patient advocacy in a changing world and expressed appreciation for the global community’s commitment to patient-centered solutions.
A celebration of collaboration and shared dedication: More than just a meeting, the Asia-Pacific Regional Gathering was a celebration of collaboration, dialogue and a shared commitment to improving healthcare for patients worldwide. It provided a fertile ground for making connections, sharing best practices, and exploring solutions that resonate across borders.
The event aims to foster a global dialogue on patient safety, quality healthcare and advocacy. We believe that our active participation will also contribute greatly to your own understanding of the critical issues surrounding patient engagement in healthcare.
The number of rare diseases reached 432 types.
The third medical commission of Iran’s Rare Diseases Foundation was held on Sunday, November 7, with the confirmation of 10 other rare diseases.
According to the public relations report of the Rare Disease Foundation, these diseases include:
CoQ10 deficiency
Angelman syndrome
Rohhad syndrome (IBS)
Cohen-Gibson syndrome
Cushing’s syndrome
Craniosynostosis
Macrophage activation syndrome (MAS)
Hereditary primary hypomagnesemia
Giant Axonal Neuropathy
Hypophosphatasia (HPP).
This commission was attended by Dr. Yaser Davodian, Chairperson of Iran Rare Diseases Foundation, Dr. Hamidreza Edraki, CEO of the Foundation and member of the academic faculty of Shahid Beheshti University, Dr. Mehdi Norouzi, Vice President of Research of the Rare Diseases Foundation, Dr. Ali Tagavi, a member of the Scientific Council, and Dr. Mohammad Haqshenas. Internist, Dr. Shadab Salehpour, pediatric endocrinologist and metabolism specialist, Dr. Siamak Abdi, neurologist,
Dr. Hossein Afra, Vice President of Medicine, Dr. Tayebe Khamsi, Vice President of Education, Somaye Hasanpour, Vice President of Rare Diseases, and Roya Tavakoli, responsible for International Affairs of the Foundation, were held.
The US Food and Drug Administration (FDA) has approved zilucoplan (Zilbrysq, UCB) for the treatment of generalized myasthenia gravis (gMG) in adults who are antiacetylcholine receptor (AChR) antibody positive.
Zilucoplan is the first once-daily, subcutaneous, targeted C5 complement inhibitor for gMG for self-administration, the company said in a news release announcing approval.
gMG is a rare, chronic disease that affects almost 200,000 patients in the United States, the European Union, and Japan.
Patients with gMG can suffer a variety of symptoms, including drooping eyelids, double vision, and difficulty swallowing, chewing, and talking.
They can also experience severe life-threatening weakness of the respiratory muscles. In about 80% of patients, the condition progresses to generalized muscle weakness.
Clinically Meaningful Improvement
The FDA approval of zilucoplan for gMG was based on safety and efficacy results from the phase 3 RAISE study, published in The Lancet Neurology in May.
The study randomly allocated (1:1) 86 adult patients who were AChR antibody positive with gMG to receive daily subcutaneous injections of zilucoplan 0.3 mg/kg or placebo for 12 weeks.
“Zilucoplan treatment showed rapid and clinically meaningful improvements in myasthenia gravis-specific efficacy outcomes, had a favorable safety profile, and was well tolerated, with no major safety findings,” the investigators reported.
The most common adverse reactions (≥10%) among patients with gMG were injection site reactions, upper respiratory tract infection, and diarrhea.
“For people with gMG, the unpredictable nature of the severity and frequency of symptoms can be debilitating and can have a substantial impact on many aspects of their day-to-day lives,” lead investigator James F. Howard, MD, distinguished professor of neuromuscular disease, the University of North Carolina at Chapel Hill School of Medicine, said in the release.
“Zilucoplan demonstrated rapid improvements in gMG symptoms at Week 12, with differences seen as early as one week, and provides a new treatment option for a broad population of AChR antibody-positive gMG patients,” Howard added.
In June, the FDA approved rozanolixizumab (Rystiggo), also from UCB, for the treatment of adults with gMG who are positive for AChR or anti-muscle-specific tyrosine kinase (MuSK) antibody, as reported by Medscape Medical News.
Rozanolixizumab is a subcutaneous-infused humanized IgG4 monoclonal antibody that binds to the neonatal Fc receptor, reducing the concentration of pathogenic IgG autoantibodies.
“This is an important development for the community because, with more FDA-approved treatments for generalized myasthenia gravis, physicians have additional tools to treat this disease in individualized ways that are the right fit for each individual patient,” Samantha Masterson, president and CEO of the Myasthenia Gravis Foundation of America, said in the news release.


The providing and editing of the national rare diseases document is one of the most vital activities of the Rare Diseases Foundation, and in this regard, the Rare Diseases Foundation of Iran, with a lot of endeavor and follow-up, and with the participation and cooperation of the professors and expertise of the Foundation and other universities of medical sciences, prepared and compiled the National Document of Rare Diseases of Iran, which after being approved by the Secretariat of the Supreme Council of Health and Food Safety and the cooperation and collaboration of the honorable officials of that ministry and other related institutions, was finally approved by the honorable president and announced. Undoubtedly, the declaration of this document can be a very huge and effective step in determining the duties of the institutions and beneficiaries related to these patients, providing services to rare patients and their families, and solving the problems of these patient respectfully


















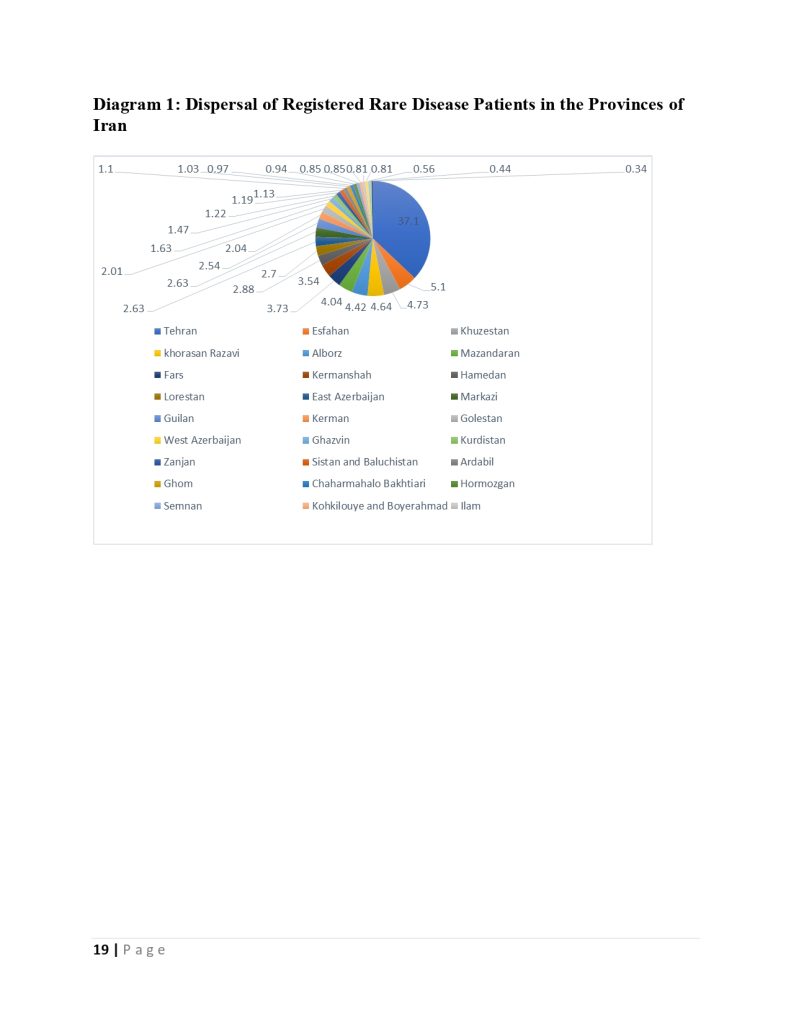
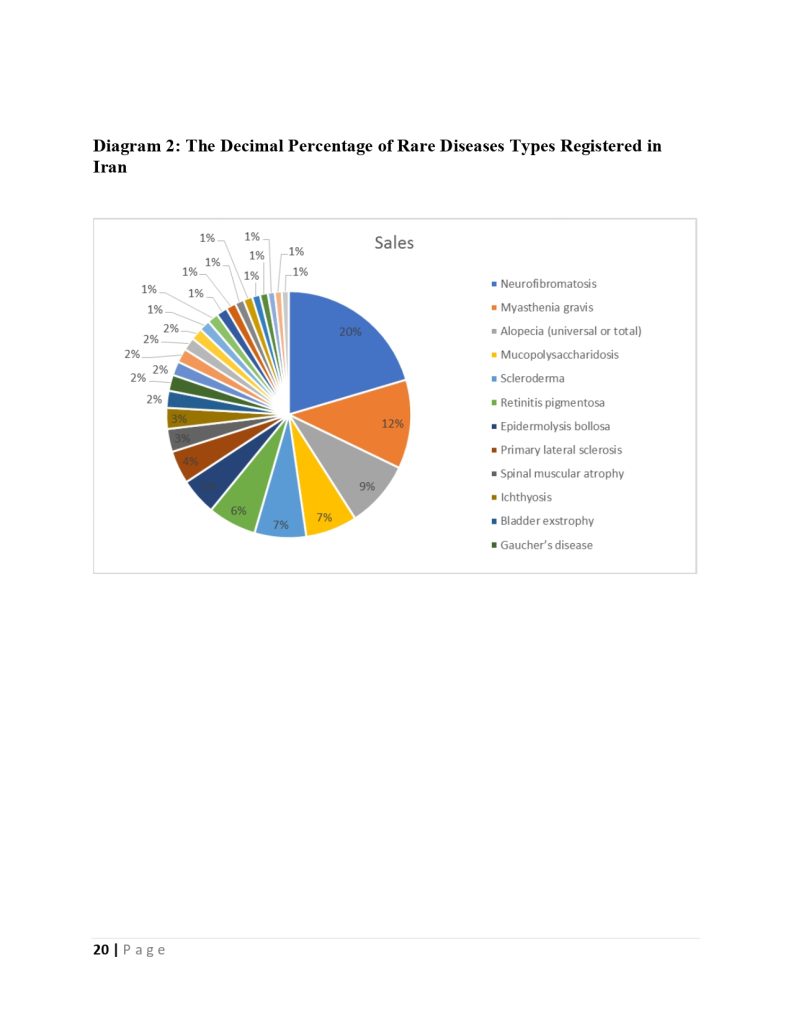
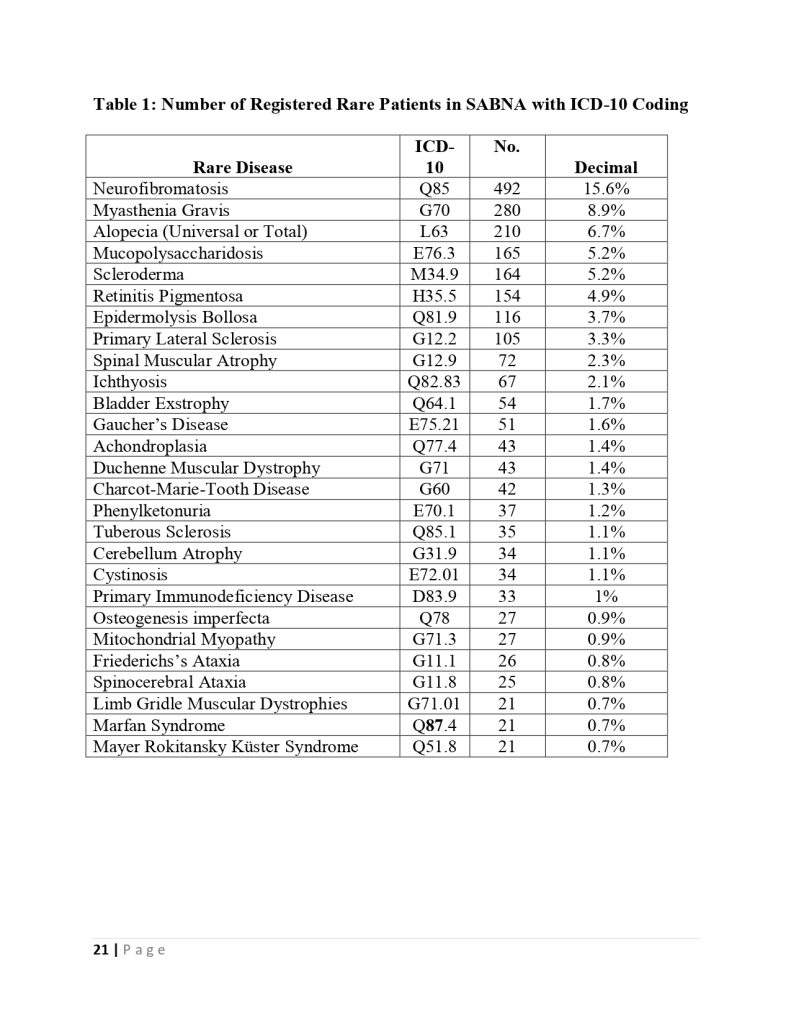
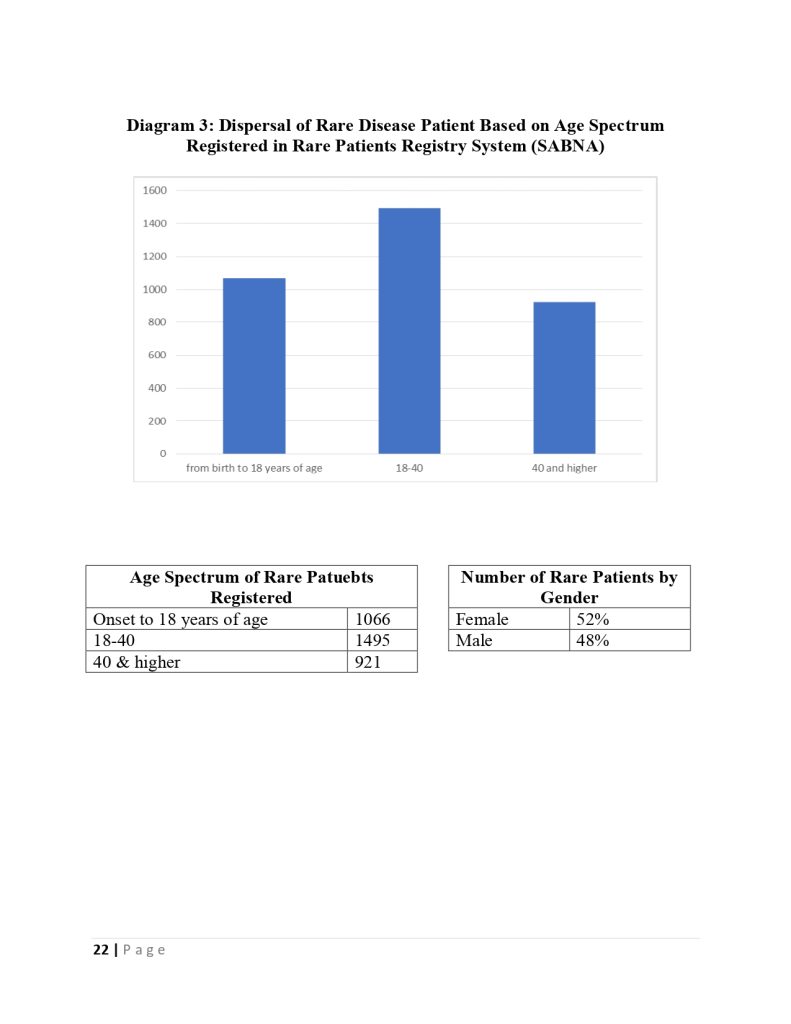























The deputy of insurance and services of the iran health insurance organization declared :we cover one million and thousand patients in the fund for rare and hard treatment diseases.
In report of mehr news agency ,Mehdi rezaei,
According the insurance coverage of rare and hard treatment patients, announce : 1,600 billion tomans have been paid in the first 4 months of this year to cover the costs of rare and hard treatment patients .
Most of services are provided in the pharmaceutical, medical radiation and laboratory services, 75% of which are related to the pharmaceutical sector .
Rezaei added: 63 service packages have been prepared in this fund for these patients, who have received the most services in the rare and hard treatment patients fund, respectively , for cancer , MS, AND HEMOPHILIA